Therapeutics
Antiviral Drug Discovery and Development Programs
Arisan Therapeutics has an ongoing Phase 1 clinical program for the development of a broad spectrum arenavirus / Lassa virus antiviral (ARN-75039) and earlier stage programs targeting Ebolavirus (ARN-75092) as well multiple novel chemical series with broader spectrum filovirus activity.
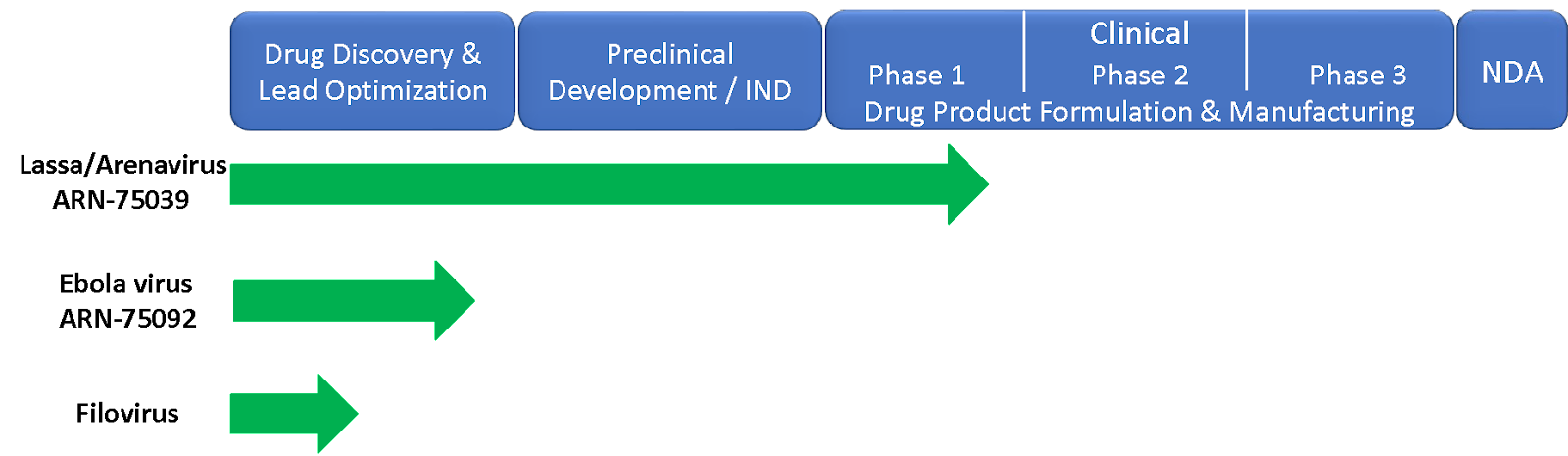
Arenavirus
 At least seven arenavirus species including Lassa virus are associated with severe arenavirus hemorrhagic fevers (AHFs) in humans. While there are no approved drugs or vaccines for the treatment of Lassa (or other AHFs) an estimated 300,000 infections and 5000 deaths occur annually from Lassa virus alone. In association with the Defense Threat Reduction Agency (DTRA), Battelle Memorial and the National Institute for Allergy and Infectious Disease (NIAID), Arisan Therapeutics has identified and is currently evaluating ARN-75039 in Phase 1 clinical studies to provide a potent, convenient and urgently needed oral broad-spectrum AHF / Lassa therapeutic.
At least seven arenavirus species including Lassa virus are associated with severe arenavirus hemorrhagic fevers (AHFs) in humans. While there are no approved drugs or vaccines for the treatment of Lassa (or other AHFs) an estimated 300,000 infections and 5000 deaths occur annually from Lassa virus alone. In association with the Defense Threat Reduction Agency (DTRA), Battelle Memorial and the National Institute for Allergy and Infectious Disease (NIAID), Arisan Therapeutics has identified and is currently evaluating ARN-75039 in Phase 1 clinical studies to provide a potent, convenient and urgently needed oral broad-spectrum AHF / Lassa therapeutic.Filovirus
 Filoviruses include a number of Ebolavirus species including Zaire (EBOV), Sudan (SUDV) and Bundibugyo (BDBV), as well the related Marburg virus (MARV), associated with severe human hemorrhagic fevers endemic to Africa, and for which there are currently no approved convenient oral therapeutic treatments. In association with Chemical and Biological Defense Program (CBD) and the National Institute for Allergy and Infectious Disease (NIAID) Arisan identified a chemical series, including a lead compound (ARN-75092) of Ebolavirus inhibitors. Additional discovery efforts have recently identified several novel broad spectrum filovirus chemical series, through structure-based rational drug design and computational chemistry, for optimization and further development.
Filoviruses include a number of Ebolavirus species including Zaire (EBOV), Sudan (SUDV) and Bundibugyo (BDBV), as well the related Marburg virus (MARV), associated with severe human hemorrhagic fevers endemic to Africa, and for which there are currently no approved convenient oral therapeutic treatments. In association with Chemical and Biological Defense Program (CBD) and the National Institute for Allergy and Infectious Disease (NIAID) Arisan identified a chemical series, including a lead compound (ARN-75092) of Ebolavirus inhibitors. Additional discovery efforts have recently identified several novel broad spectrum filovirus chemical series, through structure-based rational drug design and computational chemistry, for optimization and further development.
